SUTUREGARD® has recently announced how to document the use of HEMIGARD® ARS and SUTUREGARD® ISR devices for your complex repairs in EMA with Modernizing Medicine.
EMA has clickable boxes to simplify your documentation and coding when using the HEMIGARD® ARS and SUTUREGARD® ISR devices for your closures. The steps are easy and identifiable, below is an example for when using the HEMIGARD®.
Step 1: After documenting the excision, click “Complex Repair”
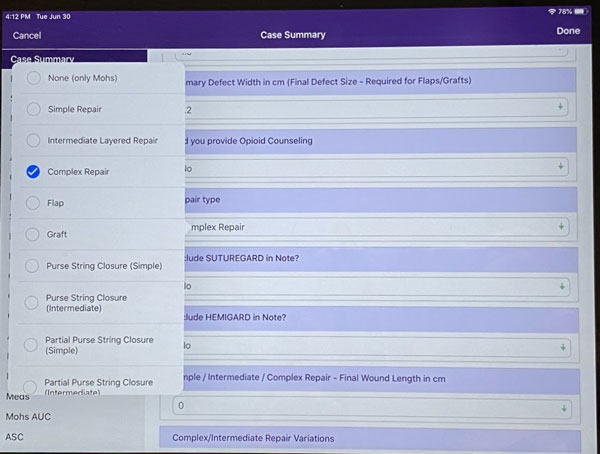
Step 2: EMA will ask if a SUTUREGARD® ISR or HEMIGARD® ARS device was used. Select “HEMIGARD”.
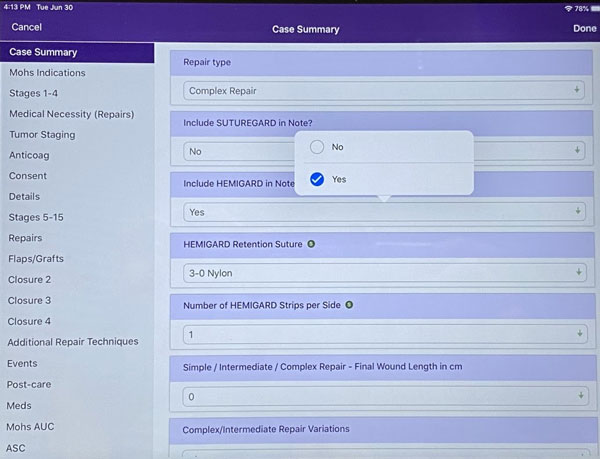
Step 3: Select the caliber of suture used for the retention suture. You can change the default using the “sticky” green ball in the box. Typically, the HEMIGARD® is used within 2-0 nylon.
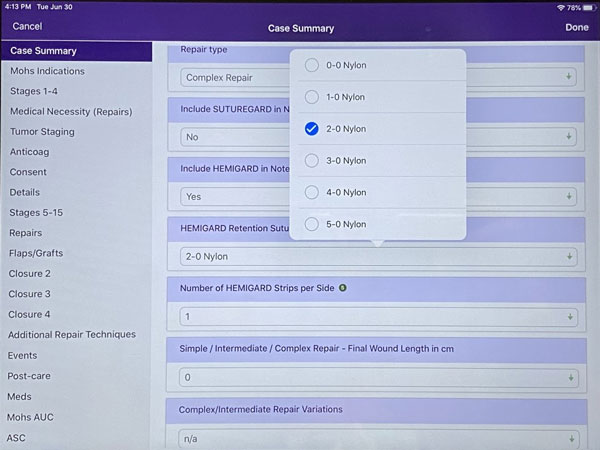
Step 4: Select the number of HEMIGARD® strips per side of the wound. One pouch of HEMIGARD® ARS devices contains two strips, one for each side of the wound. Here, we selected 1 strip per side.
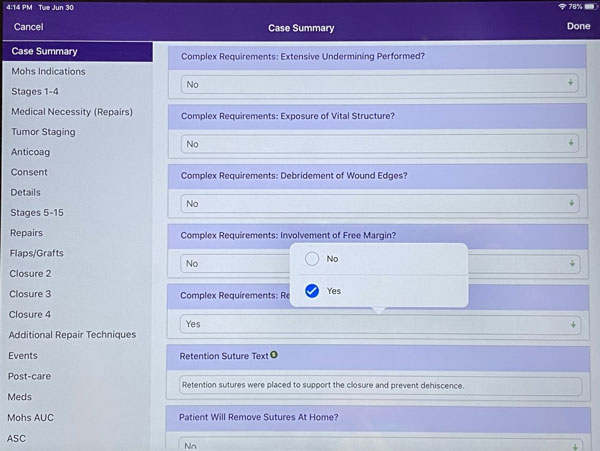
Final Step: Mark the “complex Requirement: Retention Suture Used” box as “Yes” to confirm that you placed the HEMIGARD® ARS device(s) and retention suture(s).
Based in Portland, Oregon, SUTUREGARD® Medical, Inc is a private company offering novel medical devices for surgeons to repair challenging surgical defects more simply. SUTUREGARD® medical devices are intended to provide minimal wound trauma and reduce the healing period after surgery, providing better results for patients.